Development of Autoimmunity
The breakage of self-tolerance
Autoimmune diseases are caused by an immune response against self-antigens. Tissue injury in these diseases is inflicted by autoreactive T cells and/or autoantibodies. The latter unleash effector mechanisms directly or indirectly by activating effector cells, such as myeloid or natural killer (NK) cells.
Current concepts suggest that the pathogenesis of autoimmune diseases is multifactorial and regulated by a dynamic, multilevel interplay between genetic and non-genetic factors (1-7). The latter include a large diversity of environmental and miscellaneous other biological factors. In concert, these different factors supposedly trigger and perpetuate the pathogenic processes that gradually lead to misdirected, aberrant immune responses against self and, consequently, to manifest autoimmune disease (2-5).
Pathomechanisms of Autoimmunty
The pathomechanisms initiated and driven by this panel of factors to induce and perpetuate tissue inflammation and, thus, autoimmune disease are only poorly understood. A unifying pathomechanistic model integrating the myriad of clinical and experimental observations reported for autoimmune diseases is still missing. Autoreactivity, i.e., the recognition of self-structures as foreign by the adaptive immune system, stands undisputedly at the origin of the pathogenesis of autoimmune diseases. However, autoreactivity per se is not necessarily pathological and mostly does not bear the potential to initiate disease. Thus, the pathogenesis of autoimmune diseases must involve the transition from non-pathogenic to pathogenic autoimmunity (8, 9).
Example: Emergance of a antibody-mediated autoimmune diseases
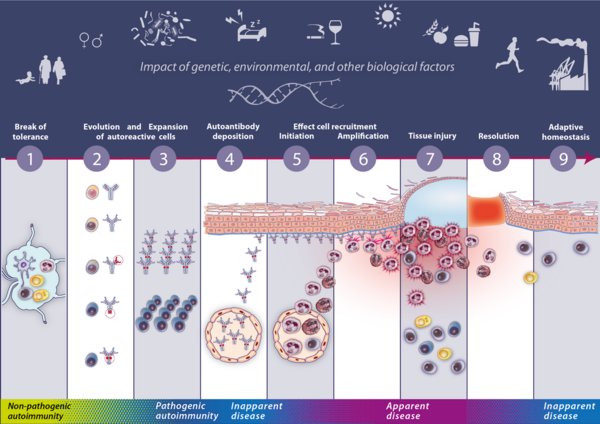
The emergence of antibody-driven, effector cell-mediated autoimmune diseases, for instance, can be considered the result of immune dysregulations at multiple levels caused by a sequence of gradual, non-linear processes. These processes can be indefinitely set on hold at any stage and are continuously regulated by multiple interacting genetic and non-genetic risk factors. The major processes of the pathogenesis can be divided in nine steps (Figure 1).
The initial break of tolerance leads to the generation of a small populations of autoreactive T and B cells without or with only low pathogenic potential (Step 1). Subsequently, evolution (Step 2) and expansion (Step 3) of autoreactive T and B cells leads to the abundant production of autoantibodies exhibiting pathogenic properties. Pathognic antibodies then accumulate in the target tissue (Step 4), leading to the initiation (Step 5) and amplification (Step 6) of effector cell recruitment into the target tissue, that inflict tissue injury (Step 7) by the execution of their effector functions. These stages are mostly followed by the complete or partial resolution of tissue inflammation (Step 8). Novel concepts suggest that the post-inflamed tissue may not be restored to the state of pre-inflammatory homeostasis but to a state of “adapted” homeostasis (Step 9), facilitating the re-initiation of effector cell recruitment in the future (3, 10, 11).
Literature
1. Cho JH, and Gregersen PK. Genomics and the multifactorial nature of human autoimmune disease. N Engl J Med. 2011;365:1612-23
2. Miller FW, Alfredsson L, Costenbader KH, Kamen DL, Nelson LM, Norris JM, et al. Epidemiology of environmental exposures and human autoimmune diseases: findings from a National Institute of Environmental Health Sciences Expert Panel Workshop. J Autoimmun. 2012;39:259-71
3. Rosenblum MD, Remedios KA, and Abbas AK. Mechanisms of human autoimmunity. J Clin Invest. 2015;125:2228-33
4. Rose AM, and Bell LC. Epistasis and immunity: the role of genetic interactions in autoimmune diseases. Immunology. 2012;137:131-8
5. Pollard KM. Environment, autoantibodies, and autoimmunity. Front Immunol. 2015;6:60
6. Inshaw JRJ, Cutler AJ, Burren OS, Stefana MI, and Todd JA. Approaches and advances in the genetic causes of autoimmune disease and their implications. Nat Immunol. 2018;19:674-84
7. Kuchroo VK, Ohashi PS, Sartor RB, and Vinuesa CG. Dysregulation of immune homeostasis in autoimmune
8. Cohen IR. Activation of benign autoimmunity as both tumor and autoimmune disease immunotherapy: a comprehensive review. J Autoimmun. 2014;54:112-7
9. Olsen NJ, and Karp DR. Autoantibodies and SLE: the threshold for disease. Nat Rev Rheumatol. 2014;10:181-6
10. Fullerton JN, and Gilroy DW. Resolution of inflammation: a new therapeutic frontier. Nat Rev Drug Discovery. 2016;15:551-67
11. Feehan KT, and Gilroy DW. Is Resolution the End of Inflammation? Trends Mol Med. 2019;25:198-214